Abstract
Hematopoietic stem cell transplantation (HCT) is an effective therapeutic option for many cancers and disorders of immune dysfunction. However, acute graft versus host disease (GVHD) remains a morbid complication of HCT, caused by donor T cells attacking the recipient's organs. GVHD can be severe, resulting in multiorgan failure and death. Current immunosuppressive therapies remain marginally effective. The DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is emerging as a master regulator of the immune system. It is required for lymphocyte maturation and immune system diversity due to its role in V(D)J recombination. Our published data and others have determined that DNA-PKcs is required for cytokine expression, namely IL2, IL4, IL6, IL10, IFN, and TNF. Therefore, we hypothesized that DNA-PKcs inhibitors could serve as effective immunosuppressants for transplant patients to prevent rejection, which we tested in a murine allogeneic skin graft model. Ear skin from Balb/c mice was transplanted onto the backs of C57bl/6 or DNA-PKcs null mice (KO). C57bl/6 mice were treated with saline, FK506, or NU7441, a potent and selective DNA-PKcs inhibitor. Treatment with NU7441 significantly reduced graft rejection compared to both saline and FK506. Similar levels were observed in the KO mice, with less than 10% rejection after 10 days when a majority of the controls were completely rejected. These results led us to hypothesize that DNA-PKcs is required for cytotoxic activity of T cells, highlighting the potential use of DNA-PKcs inhibitors to prevent and/or treat GVHD in HCT patients .
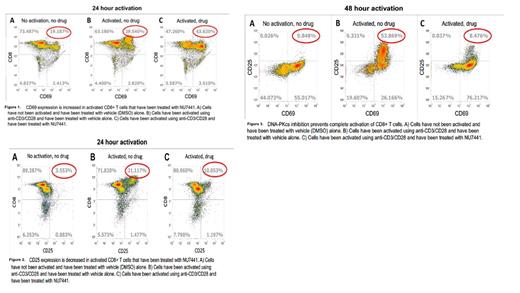
In order to evaluate the role of DNA-PKcs on T cell activation, we isolated CD8+ T cells from mouse spleens using negative selection via magnetic column. The T cells were then activated with anti-CD3/CD28 for 24-48 hours in the presence of NU7441 or vehicle (DMSO), and the cells were analyzed by flow cytometry. A fully activated T cell expresses both CD69 (early) and CD25 (later) on the surface. T cells that had been activated with NU7441 had a higher expression of CD69 and lower expression of CD25 (figures 1&2). These results are even more pronounced after 48 hours, with a much lower percent of cells expressing both CD69 & CD25 compared to T cells that had been activated in the presence of DMSO alone (figure 3). This suggests that DNA-PKcs plays a role in the activation pathway of cytotoxic T cells - the T cells are blocked from becoming completely activated as evidenced by a significant decrease in cells expressing both CD69 and CD25. If DNA-PKcs is required for the complete activation of CD8+ T cells, this may have therapeutic implications in the prevention of GVHD as cytotoxic T cells are required to cause the pathophysiologic manifestations of GVHD.
We then addressed whether DNA-PKcs affects the expression of cytotoxic genes in CD8+ T cells. The T cells were isolated and activated in the presence of NU7441 or vehicle as described above. Total RNA was then isolated from the activated T cells, and qPCR was performed to assess the difference in cytotoxic gene expression. Several genes required for cytotoxic function of CD8+ T cells, including perforin-1, granzyme, and eomes. IL2Ra is the gene coding the CD25 surface marker/IL2 receptor. CTLA4 and TGFb are associated with immune tolerance. A statistically significant decrease in gene expression was seen when treating with NU7441 in the expression of granzyme (p-value 0.01), eomes (p-value <0.01), and IL2Ra (p-value 0.04). While other data was not statistically significant, an interesting trend was seen in increased expression of TGFb. Overall, the significant data suggests that DNA-PKcs plays a role in the expression of certain cytotoxic genes, and may lead to overall decreased cytotoxic activity, perhaps by both decreasing expression of certain genes required for cytotoxic activity/cytokine production as well as increasing the expression of other genes involved in immune tolerance. Both of these effects could potentially lead to a favorable outcome in prevention of acute GVHD.
This data serves as an excellent proof of concept for testing the hypothesis that DNA-PKcs inhibitors can prevent acute GVHD in an animal model. This preclinical model would potentially lay the foundation to move into preclinical studies and eventually phase I clinical trials to prevent and/or treat GVHD, creating a new therapeutic option for a morbid complication for HCT patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal